Adipose Stromal Vascular Fraction Restores Coronary Microvascular Flow-Mediated Dilation in Aging Female Rats via Enhanced Peroxynitrite Signaling
Evan P. Tracy, B.S., Jason Beare, M.S., Gabrielle Rowe, M.S. Amanda Jo LeBlanc, Ph.D.
Cardiovascular Innovation Institute, Department of Physiology, University of Louisville, Louisville, KY


Abstract
Introduction: Coronary Microvascular Disease (CMD) presents in a majority of aging post-menopausal women and is defined by hyperconstricted tone of the microvessels. CMD can lead to chronic ischemia exacerbated by reduced perfusion with aging. Flow-mediated dilation (FMD) primarily contributes to the regulation of physiologic vascular patency via endothelial shear stress, but the signaling mechanism of FMD alters throughout the lifespan from vasoprotective nitric oxide (NO) in youth to the less efficacious hydrogen peroxide (H2O2) in advancing age. Reactive Oxygen Species (ROS), including H2O2, suppress NO signaling, cause cellular damage and are pro-atherogenic. Adipose Stromal Vascular Fraction (SVF) represents a heterogenous population of cells that have antioxidant and regenerative properties. We hypothesize that FMD is reduced with age, but aged rats treated with SVF will restore FMD signaling via increases in NO and decrease ROS at baseline and with flow. Methods: Isolated coronary microvessels from young (4 months), old (24 months), or old + SVF (OSVF) (tail vein injection 4 weeks prior to sacrifice) from female Fischer 344 rats were assessed via pressure myography. FMD was assessed at flow rates of 5, 10, 15, 20, and 25 μL/min at physiologic pressure (45 mmHg) and temperature (37°C). Mean Fluorescence Intensity (MFI) of NO, H2O2, superoxide (O2-), peroxynitrite (ONOO-) and antioxidant glutathione (GSH) were assessed via intraluminally-infused fluorescence dyes (2,3-diaminonapthalene (DAN), mitopy1, mitosox, biotracker 515, and SEMKUR-IM, respectively) at baseline and during flow at 10 and 25 μL/min. Results: Percent relaxation to intraluminal flow (FMD) is reduced with aging and restored to youthful levels by SVF (Figure 4a). At baseline, H2O2 and ONOO- are significantly increased with aging compared to young and OSVF (Figure 2a-d). O2- is reduced in OSVF compared to aging alone (Figure 2e-f). NO and GSH are significantly reduced with aging and increased slightly with SVF (Figure 2g-j). Intraluminal flow preferentially increases MFI of NO in youth, H2O2 in aging, and ONOO- in OSVF (Figure 3). These data correspond functionally as FMD was significantly attenuated in youth via L-NAME inhibition of NO production (Figure 4b), inhibited in OSVF via uric acid scavenging of ONOO- (Figure 4d), but was unaffected by reducing H2O2 signaling via catalase in aging controls (Figure 4c). Conclusions: FMD is abrogated with aging, contributing to CMD hyperconstriction associated with an increase in ROS and loss of NO and GSH. FMD can be restored with therapeutic SVF associated with an increase in ONOO- signaling in response to intraluminal flow. Although ONOO- is considered a ROS, it could be considered a NO reservoir potentially inducing FMD as a NO donor since O2- and NO must combine to form ONOO-. Alternatively, ONOO- can elicit FMD as an Endothelium-Derived Hyperpolarizing Factor. Future directions will differentiate between these alternative mechanisms. SVF represents a plausible future clinical direction to alleviate vascular oxidative stress and restore microvascular patency and perfusion.Introduction
- Advanced age is a significant risk factor for cardiovascular disease. In post- menopausal women, Coronary Microvascular Disease (CMD) leads to vascular hyperconstriction leading to micro-ischemia, chronic angina, and increased risk of Major Adverse Cardiac Events.
- Currently, no treatment holistically addresses the multi-factorial etiology of small artery dysfunction, including oxidative stress, endothelial dysfunction, and pathological smooth muscle tone.
- Injected SVF has significant point of care potential in advancing age, as it resides in vascular walls, reduces vasomotor tone, improves coronary flow reserve, and reverses diastolic dysfunction. SVF contains microvascular endothelial cells, perivascular cells, fibroblasts, mesenchymal cells, regulatory and natural killer T-lymphocytes, B lymphocytes, dendritic cells, and macrophages.
Methods
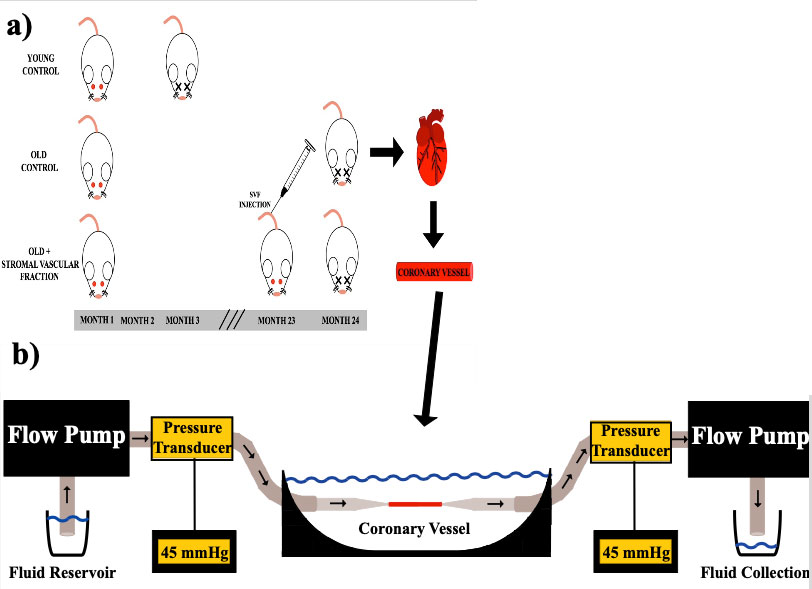
Figure 1: Experimental setup.
Rats were sacrificed at 3 and 24 months for YC and OC/OSVF, respectively. SVF was injected at 23 months into the tail vain of OSVF rats a). Coronary arterioles were incorporated into a pressure myography system b). Intraluminal pressure was 45 mmHg at 37°C. Intraluminal diameter changes over time were monitored Images of tone c), dilation d), and constriction e). Experimental Setup f).Results
- Aging increases baseline ROS (hydrogen peroxide, peroxynitrite, & superoxide) while decreasing nitric oxide and glutathione (Figure 2a-j). SVF therapy reduces ROS and restores glutathione (Figure 2a-j).
- Intraluminal flow leads to nitric oxide production in youth, hydrogen peroxide in aging, shifting to peroxynitrite with SVF therapy (Figure 3a-d). Glutathione is diminished with flow in aging alone (Figure 3e).
- Flow-Mediated Dilation (FMD) is attenuated in aging, restored by SVF (Figure 4a).
- FMD is blocked in youth by L-NAME, in OSVF by uric acid and L-NAME, and is not altered in aging by inhibition or scavenging (Figure 4b-d).
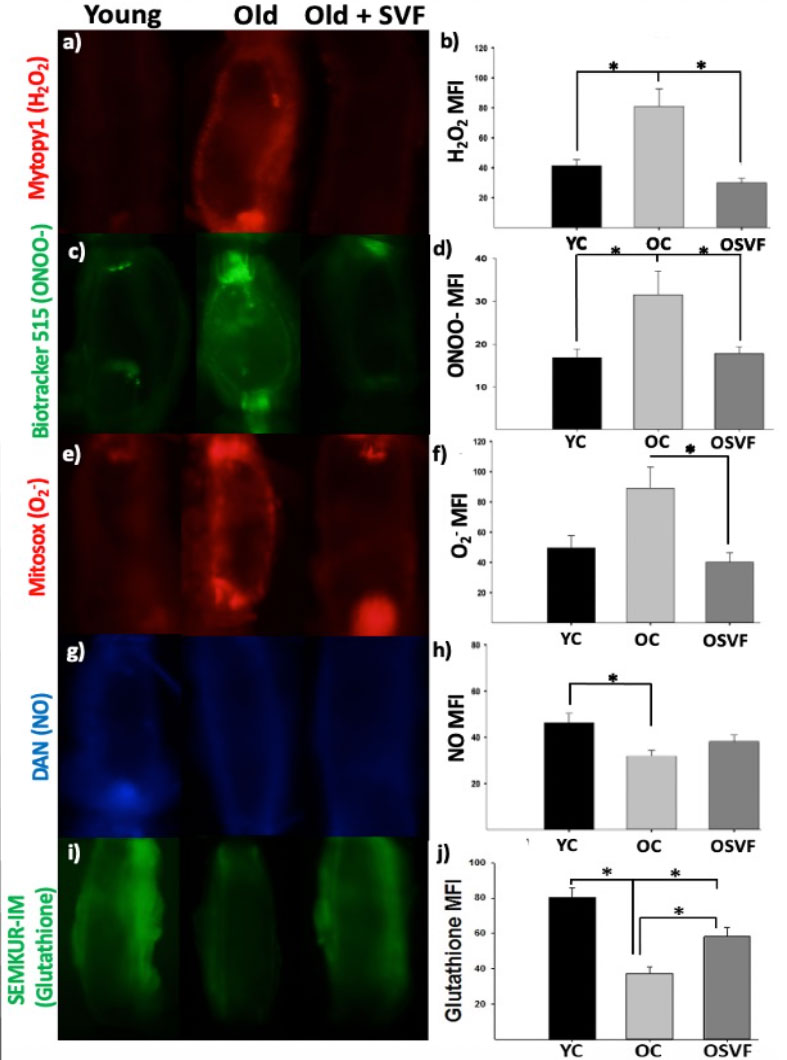
Figure 2: Baseline Mean Fluorescence Intensity (MFI) for Reactive Oxygen & Nitrogen Species and Antioxidant Glutathione.
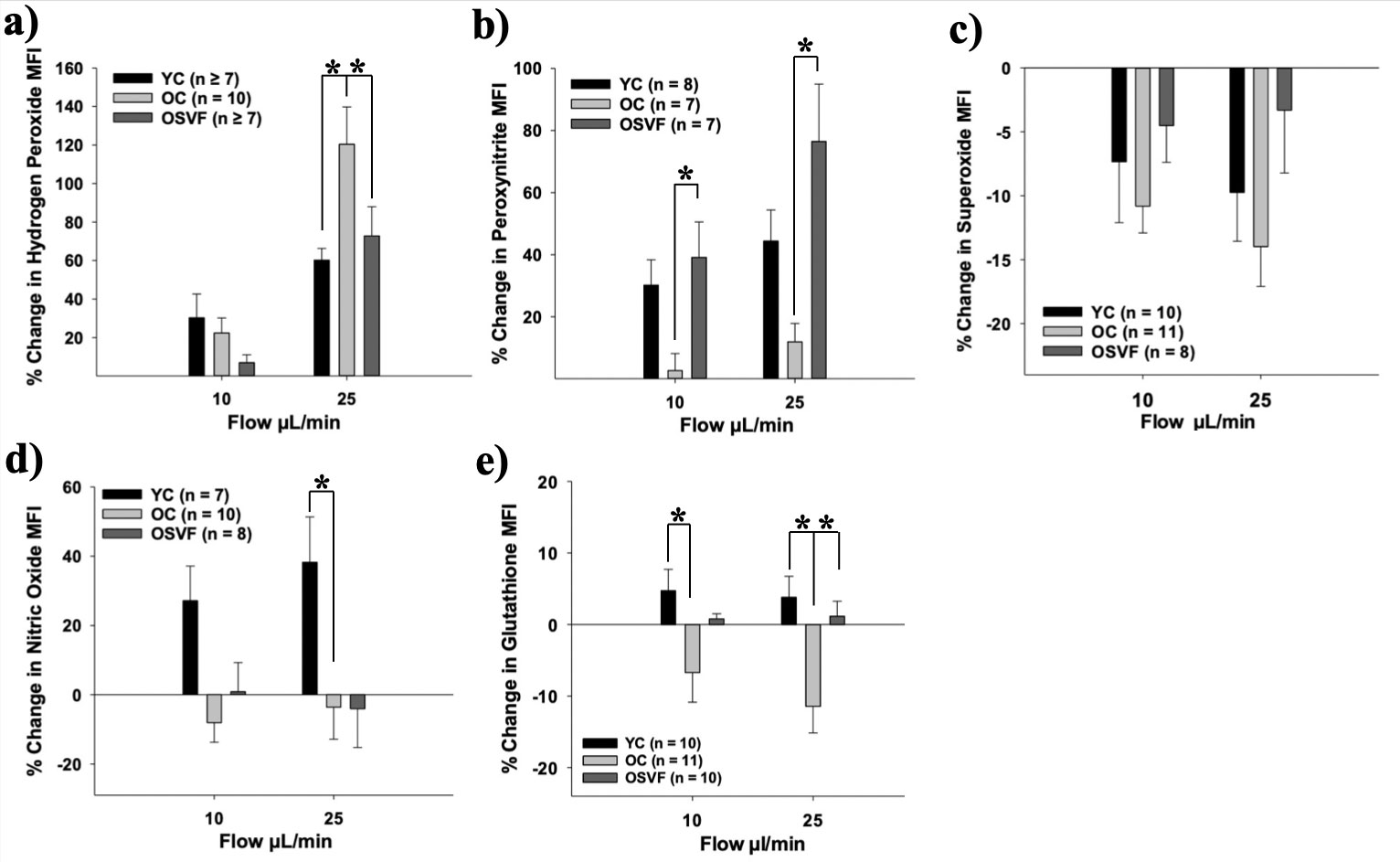
Hydrogen peroxide (H2O2), peroxynitrite (ONOO-), and superoxide (O2-) accumulation is increased with aging (OC), reversed to youthful (YC) levels by SVF therapy (OSVF) (a-f). With aging, nitric oxide (NO) bioavailability is diminished but is not fully restored with SVF therapy (g-h). Glutathione MFI is significantly reduced with aging relative to young control (i-j). SVF therapy significantly increases glutathione MFI, albeit not to youthful levels. Significance determined as p < .05 from Two-Way Repeated Measures ANOVA with Bonferroni post-hoc analysis.Figure 3: Flow-Mediated Changes in MFI
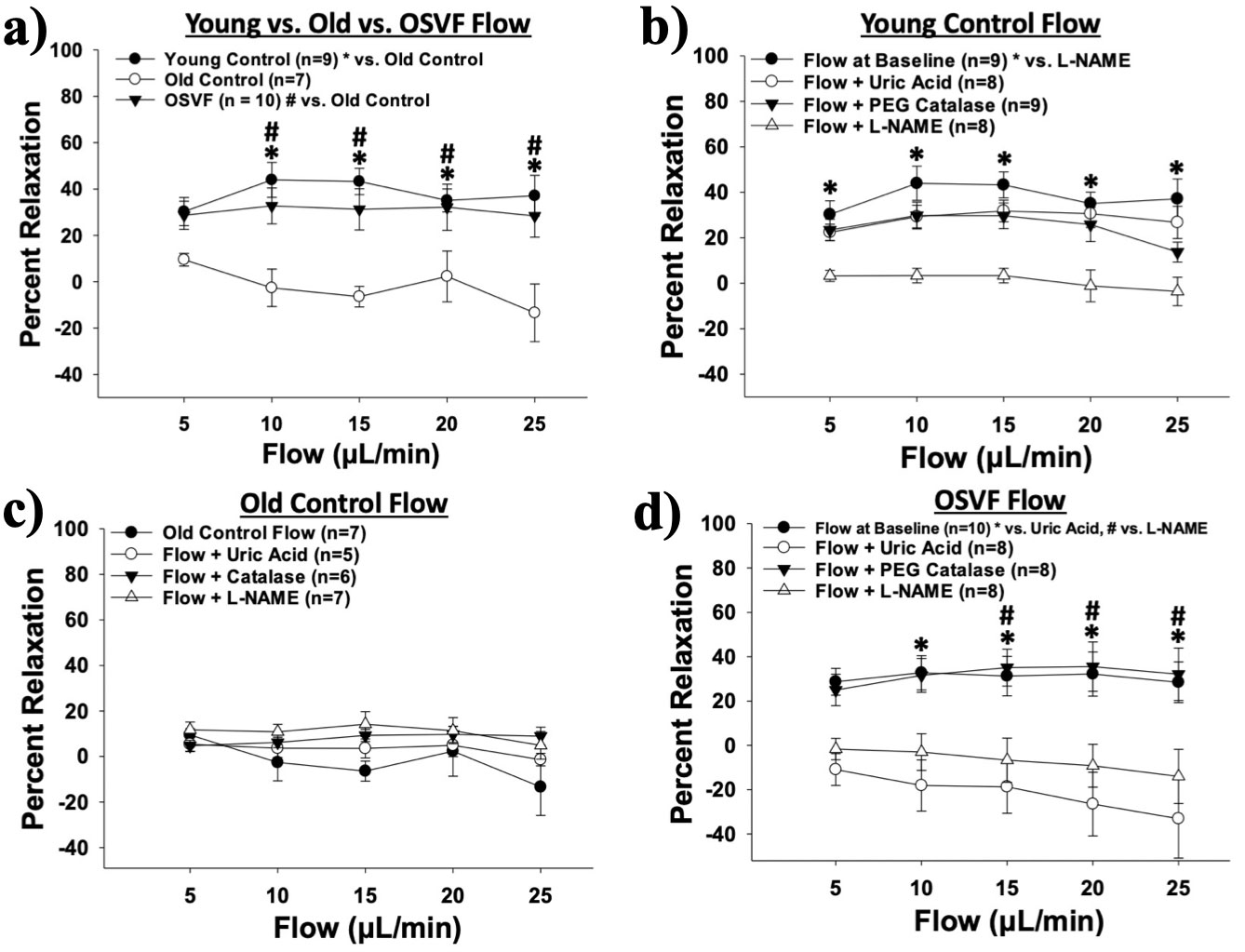
Flow induces greatest hydrogen peroxide production in aging (OC) relative to young (YC) or OSVF (a). With SVF therapy, flow mediates greater peroxynitrite production relative to young or old (b). Superoxide production with flow is not different between groups (c). Flow in youth elicits greater nitric oxide production relative to old or OSVF (d). Flow diminishes glutathione MFI in aging (e). Significance determined as p < .05 from Two-Way Repeated Measures ANOVA with Bonferroni post-hoc analysis.Figure 4: Mediators of Flow-Mediated Dilation (FMD) Throughout the Lifespan and SVF Therapy.
Intraluminal flow elicits greater dilation in young control and old + SVF (OSVF) therapy relative to old control (a). Young control FMD is attenuated with L-NAME inhibition of eNOS nitric oxide production (b). Old control FMD is not significantly altered by any of the inhibitors/scavengers including hydrogen peroxide quenching with catalase (c). FMD is restored in OSVF and attenuated both by L-NAME and Uric Acid scavenging of peroxynitrite (d). Significance determined as p < .05 from Two-Way Repeated Measures ANOVA with Holm-Sidak post-hoc analysis.Conclusions & Future Directions
- Aging increases oxidative stress and reduces antioxidative nitric oxide and glutathione. SVF therapy reverses this oxidative stress and restores antioxidant glutathione.
- Flow-Mediated Dilation is significantly attenuated in aging, restored to youthful levels by SVF therapy
- During youth, nitric oxide is primarily responsible for the Flow-Mediated Dilation (FMD) Response as its production is increased with flow and blockade with L-NAME attenuates the FMD.
- During aging, intraluminal flow primarily leads to production of hydrogen peroxide and quenching of hydrogen peroxide by catalase does not significantly alter the FMD response, likely because the baseline response is already minimal.
- With SVF therapy, peroxynitrite is most responsible for the FMD response as its production is increased with flow and scavenging with uric acid attenuates the FMD response.
- In OSVF, inhibition of nitric oxide production also significantly attenuates the FMD response, meaning that peroxynitrite-mediated FMD is dependent on at least a minimal level of nitric oxide production enough to form peroxynitrite (which forms when nitric oxide and superoxide combine).
- In future experiments, we will determine a mechanism for how SVF mediates these alterations in the FMD signaling pathway. Potential explanations include exosome-delivery of antioxidants or changes in genetic or functional expression of pro- or antioxidants.